We all have made the observation. Sometimes it can be 75° and feel absolutely gorgeous and refreshing, other times we are dripping with sweat and miserable. The difference? Humidity. The reason? It interferes with our bodies natural air conditioning system.
The human body is built to survive at certain temperatures. If our body gets too hot, our organs start to shut down. In order to survive, we have evolved to have our own cooling system, sweat. So why does this help to cool down? It’s a concept that runs with one of the most amazing compounds on our planet – water.
Water is amazing because it is the only thing that is found in all three phases naturally on our planet. Solid as ice, liquid water, and as a gas which is called water vapor. The shift between these three phases either takes in or releases heat energy.
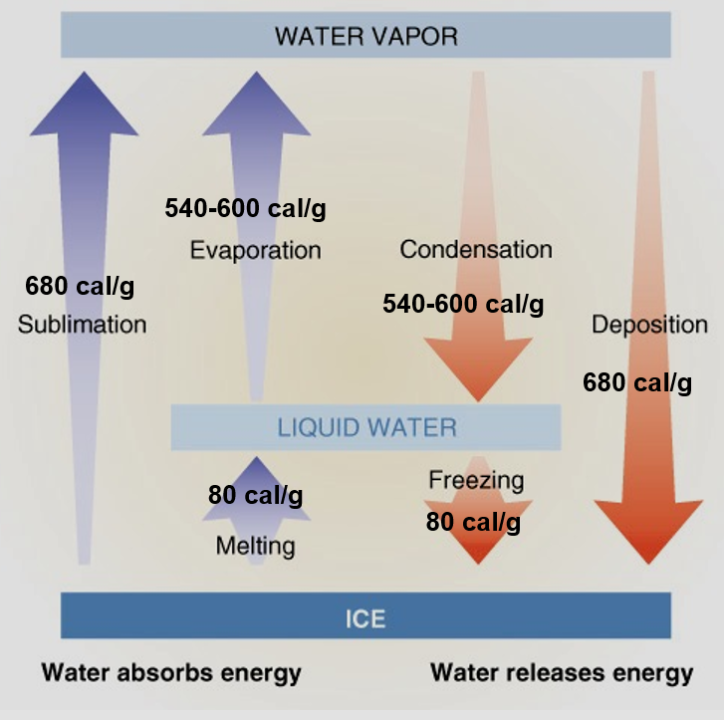
As you see in the chart above, it takes at least 500 times the energy to evaporate a gram of water than it does to raise that gram of water 1° Celsius. That’s a lot of energy! So where does that energy come from? The surrounding environment. You have made this observation by stepping out of the shower. Don’t you feel cooler when you get out compared to when you get in? The temperature of the bathroom didn’t change but the moisture and water on your skin is evaporating and stealing energy from your body. It’s this energy loss that makes you feel cool.
Now let’s circle back to your body and sweat. When your body is too warm, pores open in your skin and let water from inside of your body to the outside, in other words, sweat. This sweat then evaporates and cools your body. When it is humid, and the dewpoint is high, your sweat evaporates at a much slower rate. This means your body isn’t cooling as much so your body sweats more. On top of this, the sweat already on your skin is still there so you become sopping wet when it is humid.
Now that you understand how important sweat is, remember during hot and humid weather, stay hydrated.


